Advertisement
Fascinating Atom Facts You Need to Know
Advertisement
An atom is the fundamental building block of matter. When atoms were first discovered, they were believed to be the smallest particles that could exist. In fact, the word "atom" comes from the Greek word meaning "indivisible," because scientists thought nothing could be smaller. However, as our understanding of physics grew, we learned that atoms can be split into even smaller particles. This discovery led to the realization that splitting atoms releases energy—a process we harness through nuclear fission.
Structure of an Atom
Every atom consists of three types of subatomic particles: electrons, protons, and neutrons. Protons and neutrons form the nucleus at the center of the atom, while electrons move constantly around this nucleus. Interestingly, these subatomic particles are made up of even smaller units called quarks. Quarks are so tiny that they can't be seen directly; scientists know they exist by observing their effects on other particles. So, while atoms were once thought to be the smallest units, we now know there's a whole world inside them.
Advertisement
Ions
Most atoms are neutral, meaning they have no electrical charge. However, when an atom gains or loses electrons, it becomes an ion and carries a charge. Protons are positively charged, and electrons are negatively charged. If an atom has more protons than electrons, it's a positively charged ion called a cation. Conversely, if it has more electrons, it's a negatively charged ion known as an anion. Ions can behave very differently from their neutral counterparts. For example, sodium ions and chloride ions combine to form table salt, but neutral sodium atoms can burst into flames when they touch water. Similarly, neutral chlorine atoms can form dangerous compounds that require evacuations during accidents involving chlorine gas.

Advertisement
The Big Bang
About 13.7 billion years ago, the Big Bang gave birth to our universe. In the very first second, the universe expanded and doubled its size at least 90 times. After just ten-millionths of a second, quarks and electrons began to form and spread throughout the cosmos. Approximately three minutes after the Big Bang, protons and neutrons combined to create nuclei. Today, researchers are trying to recreate conditions similar to the Big Bang using powerful particle colliders. They hope to learn more about matter and explore possibilities like alternate realities and dimensions.
Advertisement
The First Atoms
The first atoms formed around 380,000 years after the Big Bang. It took that long for the universe to cool enough for electrons to slow down and be captured by nuclei, forming atoms. Hydrogen and helium, the lightest atoms, were the first elements to emerge. These elements became the building blocks for stars and galaxies, setting the stage for the complex universe we see today.
Advertisement
Supernovas
While the earliest atoms were light, heavier atoms were forged in the hearts of stars. When certain stars die, they explode in dramatic events known as supernovas. These explosions release so much energy that they can briefly outshine entire galaxies. The immense force generated by supernovas disperses heavy atoms throughout the universe. This scattering of elements is crucial because it seeds new star systems and planets with the materials necessary for life.

Advertisement
Forces in Atomic Nuclei
Inside an atom's nucleus, protons and neutrons are held together by a strong nuclear force. However, in some atoms, this binding force isn't strong enough to keep the nucleus stable. Unstable atoms may decay by losing neutrons or electrons in an effort to achieve stability. When an atom loses or gains electrons, it becomes an ion. If it loses neutrons, it may become radioactive, emitting radiation as it seeks a more stable state. This process is essential in fields like medicine and energy production.

Advertisement
Isotopes
Isotopes are different versions of the same element that have varying numbers of neutrons. While the number of protons in an element remains constant, the number of neutrons can change, leading to different isotopes. The term "isotope" comes from the Greek words "isos," meaning "equal," and "topos," meaning "place," because isotopes occupy the same position on the periodic table. Despite having the same number of protons, isotopes can have different physical properties and levels of stability.

Advertisement
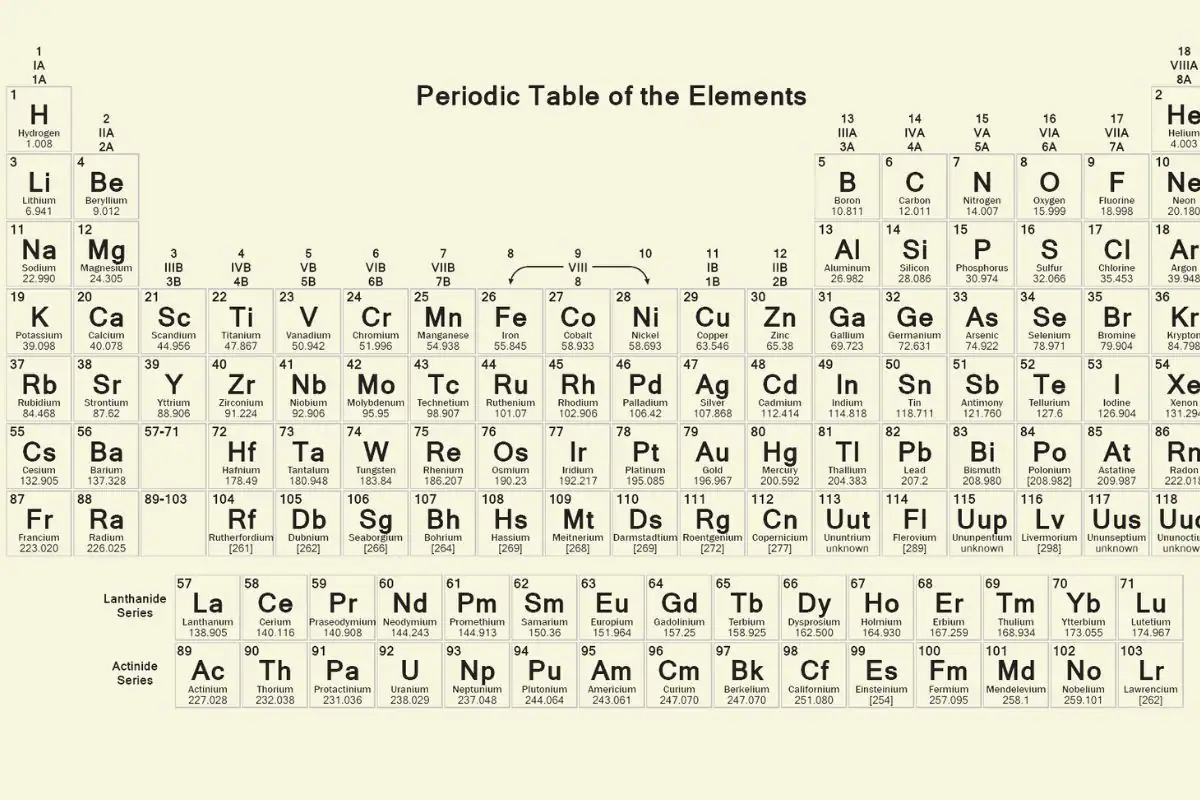
Periodic Table
The periodic table, also known as the periodic table of elements, is a chart that organizes all known chemical elements. Elements are arranged in seven rows, called periods, according to their atomic number—the number of protons in the nucleus. Every atom has at least one proton. Changing the number of neutrons creates isotopes, but changing the number of protons transforms the atom into a completely different element. The periodic table helps scientists understand the relationships between elements and predict how they will react with one another.
Advertisement
Radioactivity
When atoms are unstable due to an imbalance in their nucleus, they may try to reach a stable state by emitting particles or energy—a process known as radioactivity. This involves the atom releasing protons, neutrons, or energy in the form of radiation. The decay results in a different isotope, which may or may not be radioactive itself. Radioactivity is a natural phenomenon that has significant applications in medicine, such as in cancer treatments, and in generating nuclear energy.

Advertisement
Uranium
Uranium is the most common fuel used in nuclear reactors because it naturally occurs in the environment. Uranium-238 makes up the majority of natural uranium and isn't highly radioactive on its own. However, in a nuclear reactor, it can transform into plutonium-239, which is used as fuel. Uranium-235 is another isotope that is naturally radioactive and can be used directly in nuclear reactors and weapons without enrichment. Although only 0.7% of natural uranium is uranium-235 today, it once made up about 85% of all uranium. Its decline is due to its unstable core, which causes it to decay over time—a trait that also makes it valuable for nuclear applications.

.png)




